|
Xiamen Boson Biotech Co., Ltd.
|
Boson DOA test
| Payment Terms: | T/T, Western Union |
| Place of Origin: | Fujian, China (Mainland) |
|
|
|
| Add to My Favorites | |
| HiSupplier Escrow |
Product Detail
Intended Use
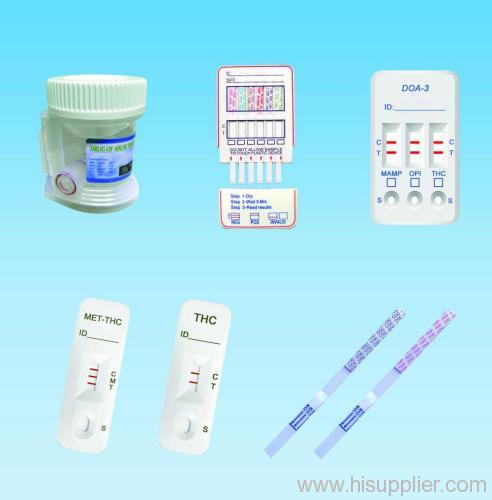
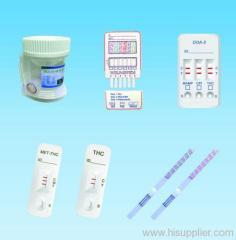
DOA Test is an immunochromatography based one step in vitro test. They are designed for qualitative determination of a drug substance.
Rapid Drug Test Card
Intended Use
DOA Test is an immunochromatography based one step in vitro test. They are designed for qualitative determination of a drug substance in human urine specimens.
Contents of Kit
DOA Test Card 25 ea
Instructions For Use 1 ea
Storage And Stability
The DOA Test should be stored at 4 to 30°C and will be effective until the expiration date stated on the package. The product is humidity-sensitive and should be used immediately after being open. Any improperly sealed product should be discarded.
Pre Cautions
1. For in vitro diagnostic and forensic use only.
2. Do not use the product beyond the expiration date.
3. Handle all specimens as potentially infectious.
4. Humidity sensitive product. Do not open foil pouch until it is ready to be tested.
5. Use a new urine specimen cup for each sample to avoid cross contamination.
Didn't find what you're looking for?
Post Buying Lead or contact
HiSupplier Customer Service Center
for help!